


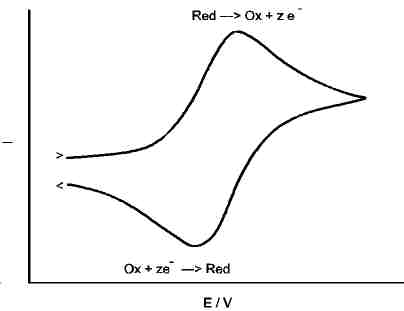
 Assume a redox reaction, its equilibrium potential shall be at Eo.
Assume a redox reaction, its equilibrium potential shall be at Eo.
To analyse it, we sweep the potential of an inert working electrode from a potential below Eo to another potential above Eo. The resulting graph may look like the one left.
Consumption of educts and diffusion effects make, that the oxidation current does not grow infinitely during the "up" sweep (anodic direction), but produces a peak. Further increase of potential does not increase the current, because the reduced species in front of the electrode is consumed.
The form of ther CV plot characterises the reaction type. There are some simple criteria:
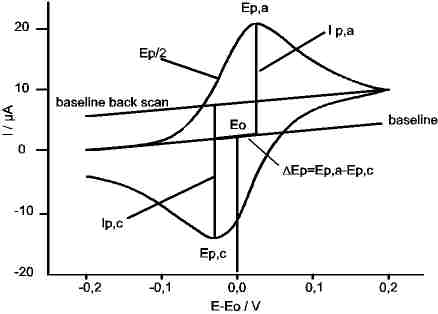
The essential features are: anodisches peak potential Ep,a and cathodic peak potential Ep,c,sand the corresponding peak currents Ip,a and Ip,c.
The criteria for sa few reactions are listed below. The simplicity of this method and the clear answers founded the popularity of CV in chemical analysis.
N.B.: For exact determination of Ep,a and Ep.c the capacitive part of the current has to be compensated.
In case, the educt is the reduced species and the CV starts in positive direction (oxidating), then:
|
Reversible |
Quasir - eversible |
Irreversible |
|
DEp=59mV/z |
DEp=f(v) |
Ep moved by 30 mV/Sqr(az) when v is tenfold |
|
Ep-Ep/2 = 56.5mV/z |
|
Ep-Ep/2 =47,7 mV/az |
|
Eo=0,5(Ep,a+Ep,c) |
Eo=0,5(Ep,a+Ep,c), 0,3<a<0,7 |
- |
|
Ipa µ sqr(v) |
|
|
|
Ip,c/Ip,a=1 für alle v |
Ip,c/Ip,a=1 für alle v |
No back- peak (Ip,c) |
|
|
|
|
(v = dE/dt, in mV/s, z the number of electrons per reaction step and a is the transfer number (usually 0.5))
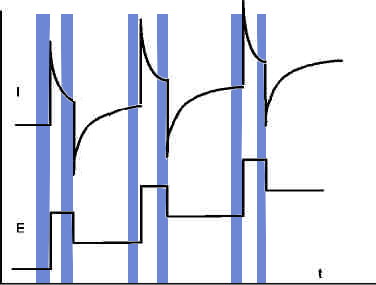
Square wave voltammetry, also referred as differential Pulse voltammetry, is the most universal method. It is applicable both for reversible and irreversible reactions. The potential is varied step-wise, and a pulse is overlaid at the beginning of each step. The current is measured both at the end of the pulses and at the end of the staircase steps.
The difference of each pair of currents (pulse and step and) is plotted vs. potential.
Especially for very small concentrations this method produces good results. The resolution threshold is in the order of pico-Mole/l .
Bank Elektronik -
Intelligent Controls GmbH
Hubertusstr. 38
D-35415 Pohlheim
Tel. +49 - 6403 - 60 98 60
Fax +49 - 6403 - 60 98 622
e-Mail: info [at] bank-ic.de