


|
Fig. 1: Universal Cell |
But it shows, that a really universal cell which delivers best result under all conditions cannot be realised. However, you will find so - called "universal cells" on the market. They got the designation because they offer convenient work for many different applications, as long as the working electrode area is in the order of some cm². They are made of glass, they have enough openings to insert different types of electrodes, thermometers, gas injectors or other auxiliary equipment.
Such a universal cell consists of a glass beaker with plane sleeve, the volume may be 0.25 l to 1 l. The lid should have at least 6 sleeve sockets to take up the electrodes and auxiliary equipment. A large sleeve for a reflow - condenser may be desired, too. Usually, the reference electrode is installed in a separate container, and connected to the main electrolyte by an electrolyte bridge.
If products are formed at the counter electrode which must not enter the main electrolyte, also the counter electrode must be installed in a separate cell compartment which is connected to the cell by a frit.
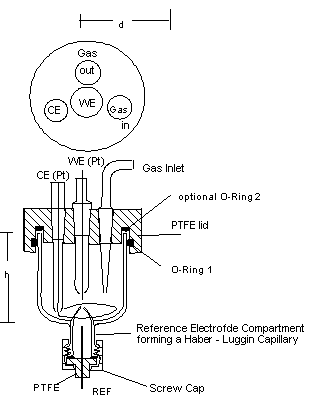
Fig. 2: 20 - ml - Cell, d about. 40 mm, h about. 35 mm.
Very fast measurements require small volumes of the cell. Instead of a calomel electrode, low-ohmic reference electrodes are required, long electrolyte bridges to the reference electrode should be avoided.
Cells, which are used for electrochemical analyses should be small to keep the required volume of the reagent to be tested as small as possible.
For cyclic voltammetry, typically small cells are desired. In order to keep the volume small, the number of bores is just the minimum for a certain measurement (may be 3 if no gas conditioning is desired). Usual CV - cells have volumes in the order of 1 ml to 100 ml.
Local corrosion of corrosion resistant alloys (CRA's) is one of the tasks where special cell designs help to minimise troubles. Local corrosion may happen as pitting, and it may happen as crevice corrosion. Crevice corrosion starts in narrow crevices, whereas pitting is observed on free surfaces. If pitting occurs, you are bound to exclude any such crevice at your working electrode. If you have to exclude some part of the working electrode from the measurement, you must apply a coating - and you will find out that you get crevices along the boundary of the coating, sometimes. It depends just on the quality of the coating, the surface preparation and the electrolyte.
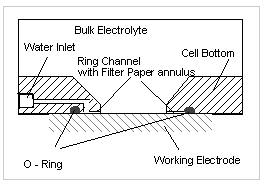
Fig. 3 Construction of the cell bottom of the Avesta - Cell
To exclude this effect, different special designs have been developed: One of it is the so - called Avesta cell, designed by Rolf Qvarfort. In this cell, an artificially produced crevice is passed at very low rate through this crevice, which prevents the material from crevice corrosion.
Sensor cells are very small cells for analytic purposes. Using special techniques, exchangeable electrodes are printed on a ceramic substrate. The ceramic substrate itself forms one wall of the cell, so volumes in the order of mm3 can be realised
Bank Elektronik -
Intelligent Controls GmbH
Hubertusstr. 38
D-35415 Pohlheim
Tel. +49 - 6403 - 60 98 60
Fax +49 - 6403 - 60 98 622
e-Mail: info [at] bank-ic.de